Abstract
Etavopivat is a small molecule activator of erythrocyte pyruvate kinase (PKR), that increases PKR activity, resulting in decreased 2,3-DPG and increased ATP in red blood cells (RBCs) of healthy volunteers (HV) and patients (pts) with sickle cell disease (SCD) (Kalfa 2019, Brown 2020). Based on initial safety, pharmacokinetic (PK) and pharmacodynamic (PD) data for HVs and pts with SCD, we performed multiple-dose studies in pts with SCD (NCT03815695): 2-wk multiple ascending dose (MD) cohorts to identify the once daily etavopivat dose that provides maximum PD activity with an acceptable safety profile and a 12-wk open label (OL) study to further characterize the safety and clinical activity at the maximum PD dose. These data are presented here.
In the completed MD cohorts, 20 pts with SCD were randomized 8:2 to receive etavopivat (300 mg, then 600 mg) or placebo (PBO) once daily for 2 wks. In the ongoing OL cohort, up to 20 pts will receive etavopivat 400 mg once daily for 12 wks. Safety assessments included adverse events (AEs), vital signs, electrocardiograms, and peripheral blood laboratory parameters. PK/PD blood sampling was performed for up to 72 h after last dose and at end of study visit. RBC function studies were performed to assess membrane deformability (Lorrca ®).
Enrollment in the MD cohorts (n=17 HbSS, n=2 HbSβ+thalassemia, n=1 HbSC) is complete and data unblinded (n=8: 300 mg etavopivat; n=8: 600 mg etavopivat; n=4: PBO). As of July 13, 2021, 11 pts (n=10 HbSS, 1 HbSC) have been treated in the OL cohort: median treatment duration was 12 (range 1-12) wks, 6 pts completed 12 wks of treatment. In MD pts, etavopivat demonstrated dose-proportional PK with overlapping PD responses (decreased 2,3-DPG and increased ATP), confirming prior results in HVs that etavopivat 400 mg once daily provides near maximal PD activity.
Etavopivat was well tolerated in both MD and OL cohorts. AEs were reported in 1 of 4 (25%) MD PBO pts, most were grade (gr) ≤3, with 1 gr 4 blood creatine phosphokinase (CPK) increase. 13 of 16 (81%) etavopivat-treated MD pts reported AEs, most were gr 1/2 and commonly (>2 pts) included sickle cell pain (n=6 [38%]), headache (n=5 [31%]), and nausea (n=3 [19%]). One pt had a serious AE (SAE) of gr 3 vaso-occlusive crisis (VOC) after completion of etavopivat (considered unrelated). In the OL cohort, AEs were reported in 7 of 11 (64%) pts who received at least 1 wk of etavopivat. AEs reported in >1 pt were headache and VOC (n=2 [18%] each). Most AEs were gr 1/2; one pt had SAEs of gr 3 acute chest syndrome and VOC (unrelated), one pt had an SAE of gr 3 deep vein thrombosis (possibly related), and one pt had an AE of gr 4 transient blood CPK increase (unrelated).
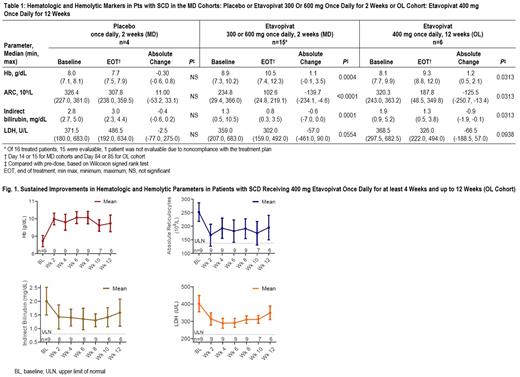
Hematologic and hemolytic parameters were significantly improved at end of treatment in both MD and OL cohorts (Table 1); 11 of 15 (73%) evaluable MD pts achieved a Hb increase ≥1g/dL over baseline (mean 1.1 g/dL, P<0.004). Decreases in absolute reticulocyte count (ARC), indirect bilirubin and lactate dehydrogenase (LDH) were observed (Table 1). These initial observations were sustained in pts receiving up to 12 wks of etavopivat in the OL cohort (Table 1, Fig. 1). Of 6 pts who completed 12 wks of etavopivat treatment, 5 (83%) achieved >1g/dL Hb increase over baseline (mean 1.39 g/dL). Reductions in ARC, indirect bilirubin and LDH were also observed. Of 9 pts on etavopivat for at least 4 wks, 8 (89%) reported an increase in Hb >1g/dL, and the highest mean Hb increase was 1.81 g/dL during active treatment.
Etavopivat-treated RBCs from MD pts (n=14) demonstrated improved functional health, including point of sickling and deformability. The improved deformability persisted up to 1 wk after etavopivat treatment in 36% of pts. Similar results were observed in the initial OL pts. Data on additional treated pts will be presented.
Etavopivat 400 mg once daily for up to 12 wks was well-tolerated, with a safety profile consistent with underlying SCD. Increases in Hb >1 g/dL were observed in 89% of pts and maintained throughout 12 wks of treatment in the majority (83%) of pts. Increased Hb and a significant reduction in ARC indicated that etavopivat enhanced survival of sickle RBCs and significantly improved the severe anemia associated with SCD. These longer-living sickle RBCs have improved membrane health that may further reduce the risk of VOCs and end-organ damage. These results support further evaluation of etavopivat in the ongoing Phase 2/3 Hibiscus Study in pts with SCD (NCT04624659).
Brown: Imara: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Novo Nordisk: Consultancy; Forma Therapeutics: Research Funding; Pfizer: Research Funding; Global Blood Therapeutics: Consultancy, Research Funding. Saraf: Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding. Idowu: Pfizer: Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Forma Therapeutics, Inc.: Research Funding; Ironwood: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kalfa: Agios Pharmaceuticals, Inc.: Other: Steering Committee, Research Funding; FORMA Therapeutics, Inc: Research Funding. Geib: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Forsyth: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Schroeder: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Wu: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Kelly: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Telen: GlycoMimetics, Inc.: Consultancy; Novartis, Inc.: Other: Data Safety Monitoring Board; Forma Therapeutics, Inc.: Consultancy, Research Funding; CSL Behring, Inc.: Research Funding; Doris Duke Charitable Foundation: Research Funding; National Institutes of Health: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal